SWIMMING POOL
BIOCIDAL AND REACH REGULATIONS
2016-01-26
REACH is a European Union regulation adopted to improve protection for human health and the environment
REACH is a European Union regulation adopted to improve protection for human health and the environment against the risks related to chemicals, while promoting the competitiveness of the chemical industry in the EU.
REACH is the acronym for "Registration, Evaluation, Autorisation and Restriction of Chemicals". The regulation came into force on 1 June 2007.
In principle, the REACH regulation applies to all chemical substances: those that are used in industrial processes, and also those that are used in our everyday life, for example in cleaning products, paints and articles such as clothing, furniture and electrical appliances. Consequently this regulation has an impact on most companies in the EU.
The REACH regulation places the burden of proof on businesses. To apply it, companies must identify and manage the risks associated with the substances they manufacture and market in the EU. They must show to the ECHA (European Chemicals Agency) how the substance can be used safely and explain the risk management measures to users.
Businesses must register their substances; to do this, they must collaborate with other companies that register the same substances.
It is the responsibility of businesses to collect information on the properties and uses of the substances they manufacture or import in quantities greater than one tonne per year. They must also assess the hazards and potential risks linked to the substance.
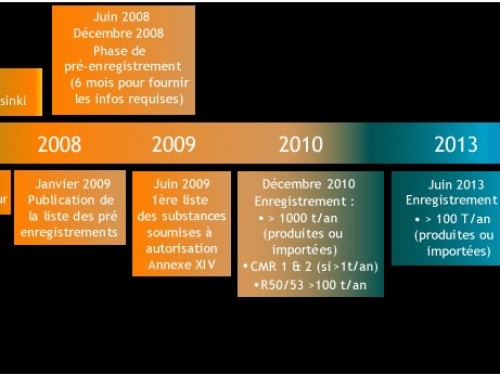
This information is communicated to the ECHA by means of a registration dossier containing the information relating to danger and, if applicable, an assessment of the risks that the use of the substance may present as well as the way of controlling them.
Registration is based on the principle of "One Substance, One Registration". This means that manufacturers and importers of the same substance must submit their registration jointly.
The ECHA receives the individual records and assesses the compliance, and then the Member States of the EU assess certain substances to meet initial concerns relating to human health or the environment. The authorities and the scientific committees of the ECHA determine if the risks relating to the substances can be managed.
REACH has an impact on a wide range of businesses in many sectors, including on those that may consider that they are not concerned by chemicals.
As a general rule, in the context of REACH, you can be considered as:
A manufacturer: If you manufacture chemicals, either for your own use or to supply them to other people (including for export); you probably take on significant responsibilities within REACH.
An importer: If you purchase any products from outside the EU/EEA, you will probably have some responsibilities in the context of REACH. They may be chemical substances such as mixtures intended for resale or finished products, such as clothing, furniture or plastic goods
A downstream user: most companies use chemicals, sometimes even without their knowledge; consequently you should check your obligations if you handle chemicals in your industrial or professional activities. You may have some responsibilities within the context of REACH.
Alongside REACH, there is a Regulation on biocidal products
The regulation on Biocidal Products (BPR, Regulation (EU) No. 528/2012) of 22 May 2012, covers the placing on the market and the use of biocidal products, which are used to protect man, animals, materials or goods against harmful organisms such as pests and bacteria, by the action of the active substances contained in the biocidal product.
Process
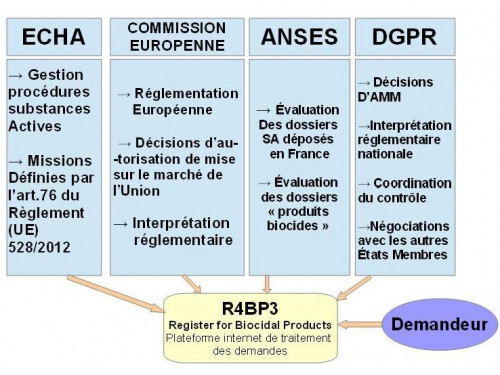
Companies need to apply for approval of an active substance by submitting a file to the ECHA.
Active Substances (AS) and Biocidal Products (BP) are classified into 22 "product types» (PT)
Companies can ask the ECHA to establish the technical equivalence of their active substances.
A fundamental and new aspect of the regulation on biocidal products is the common obligation to share information on active substances and products approved and authorised in the EU.
After the approval of an active substance, businesses wishing to put biocidal products on the market in a Member State need to seek approval for these products.
From 1 September 2015, biocidal products are no longer made available if the supplier of the AS or BP is not registered on the list (Art. 95) of the "relevant" active substances for the PT required.
Official list of suppliers, contained in Article 95 of the said Regulation Http://echa.europa.eu/information-on-chemicals/active-substance-suppliers
Hence all products containing substances and mixtures from manufacturers and/or importers not mentioned on the said list can no longer be used or marketed. It is therefore essential to ensure that the supply chain of products that you sell comply with these points.
The products concerned for Swimming pools:
Group 1: Disinfectants
These types of products do not include cleaning products that are not intended to have a biocidal effect, including washing liquids, powders and similar products.
PT2
Disinfectants and algaecidal products not intended for direct application on human beings or animals
Used to disinfect surfaces, materials, equipment and furniture which are not used in direct contact with foodstuffs or food for animals. The places of use include pools, aquariums, basin water and other water, air conditioning systems, as well as the walls and floors in private, public and industrial premises and in other places used for professional activities.
Used to disinfect air, water not used for human or animal consumption, chemical toilets, waste water, hospital waste and the ground.
Used as algaecide products for the treatment of swimming pools, aquariums and other water, as well as for curative treatment on building materials.
Used to be incorporated into textiles, fabrics, masks, paintings and other articles or materials, to produce treated articles with disinfectant properties.
To help comprehension: http://reach-info.ineris.fr/sites/reach-info.gesreg.fr/files/pdf/11028-4_REACH_maitrisez-risques-entreprises.pdf
TO SUMMARISE
Active Substance (AS)
A substance or micro-organism that exerts an action on or against harmful organisms
Biocidal product (BP)
Any substance or mixture, in the form in which it is supplied to the user, consisting of, containing or generating one or more ASs that is destined to destroy, repel, render harmless, prevent the action of, or otherwise combat any harmful organism by any means other than mere physical or mechanical action.
Treated Article (TA)
A substance, mixture or article that has been treated with one or more BPs, or in which one or more BPs have been deliberately incorporated.
A TA having a primary biocide function is regarded as a BP
PRINCIPLE
Active Substance (AS) => APPROBATION
Each substance is studied individually to prove its effectiveness and its use.
Once approved, it is added to a European Community list of "approved" ASs that can be used in biocidal products.
Biocidal Product (BP) => AUTORISATION
When all the Active Substances present in the biocidal product are added to the EC list of "approved" ASs, the persons putting the product on the market will have 2 years to apply for an authorisation for placing the product concerned on the market.
These authorisations may be national European / and multi-company (grouping of companies in the form of a GIE).
QUESTIONS OR COMMENTS?
Contact us and our team of experts. We can help with water treatment issues, product questions, or finding an Acti reseller near you.